
Simulate Correlated Progression-Free Survival and Overall Survival as Endpoints
Source:vignettes/simulatePfsAndOs.Rmd
simulatePfsAndOs.RmdProgression-free survival (PFS) and overall survival (OS) are commonly used as confirmatory endpoints in clinical trials. These two endpoints are typically highly correlated, and it is always the case that PFS OS. A reliable algorithm for simulating both PFS and OS is therefore of great interest.
Several methods have been proposed to achieve this, but many rely on latent variables or copula-based approaches, making it difficult to interpret and specify model parameters in practical settings. In this vignette, we do not aim to provide a comprehensive review of existing methods. Instead, we focus on a three-state illness-death model consisting of the following states: initial (0), progression (1), and death (2). For more details, refer to Meller, Beyersmann, and Rufibach (2019).
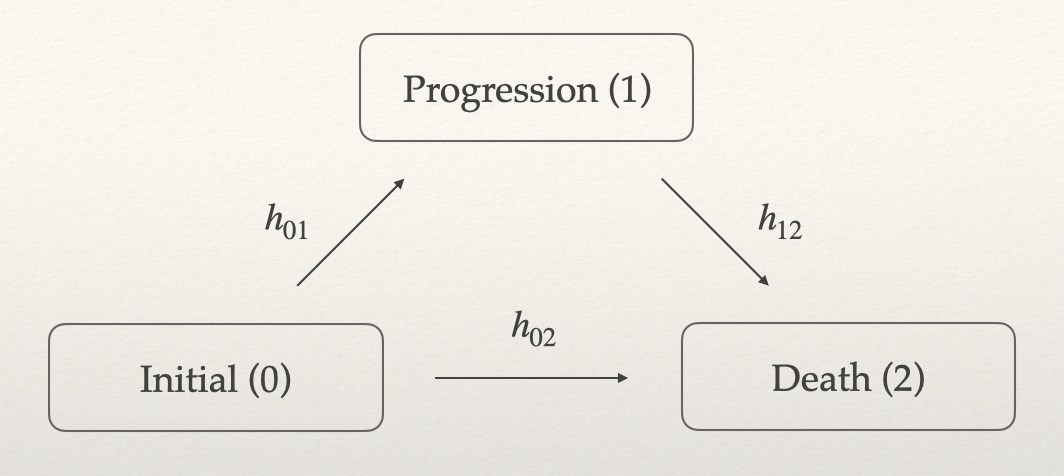
We consider the simplest case of the illness-death model, where all
transition hazards
are constant over time. A data generator of this model is implemented in
TrialSimulator::CorrelatedPfsAndOs3(), which can be used to
define endpoints in TrialSimulator::endpoint().
Marginally, the survival functions of PFS and OS are given by
and
Thus, PFS follows an exponential distribution with rate , which is also referred to as the all-cause hazard. Given the transition hazards , a patient’s trajectory can be simulated using the following algorithm:
Step 1. Generate the time to progression from an exponential distribution with rate .
Step 2. Draw a Bernoulli sample with success probability . If successful, the patient dies immediately, i.e., time to death , and the simulation ends. Otherwise, proceed to Step 3.
Step 3. Generate the time from progression to death from an exponential distribution with rate . Then, time to death , and the simulation ends.
Reparameterization
Although statistical methods exist for estimating transition hazards
when data are available (e.g., the R package simIDM), in
simulation studies for trial planning, it is common to specify these
hazard parameters based on limited information. A more intuitive way to
define PFS and OS is through their medians and correlation coefficient.
In this section, we describe a reparameterization strategy that maps the
medians and correlation to the transition hazards.
Let and denote the medians of PFS and OS, respectively. Then we have
and
Through algebraic manipulation, we obtain
This implies that, for given medians
and
,
we can perform a grid search over values of
.
For each candidate value, we compute
and
using equations (1) and (2), respectively. Then, we simulate a large
dataset using TrialSimulator::CorrelatedPfsAndOs3() and
calculate the Pearson correlation between PFS and OS. We select the
value of
(and the corresponding
)
that yields the desired correlation.
This reparameterization of the three-state illness-death model
enables us to specify correlated PFS and OS based on their medians and
correlation, which is more interpretable and practical in clinical trial
design. The TrialSimulator package provides a dedicated
function, solveThreeStateModel, to facilitate this
process.
Example
Suppose we aim to simulate PFS with a median of 5 and OS with a median of 12, targeting a correlation of 0.6 between them.
pars <- solveThreeStateModel(median_pfs = 5, median_os = 12,
corr = seq(.55, .65, by = .05),
h12 = seq(.05, .15, length.out = 50))
plot(pars)
#> corr h01 h02 h12 error
#> 1 0.55 0.0997785 0.03885093 0.08877551 0.001158721
#> 2 0.60 0.1080742 0.03055522 0.10102041 0.003826014
#> 3 0.65 0.1214222 0.01720720 0.11938776 0.004866790
#> Warning: Removed 3 rows containing missing values or values outside the scale range
#> (`geom_segment()`).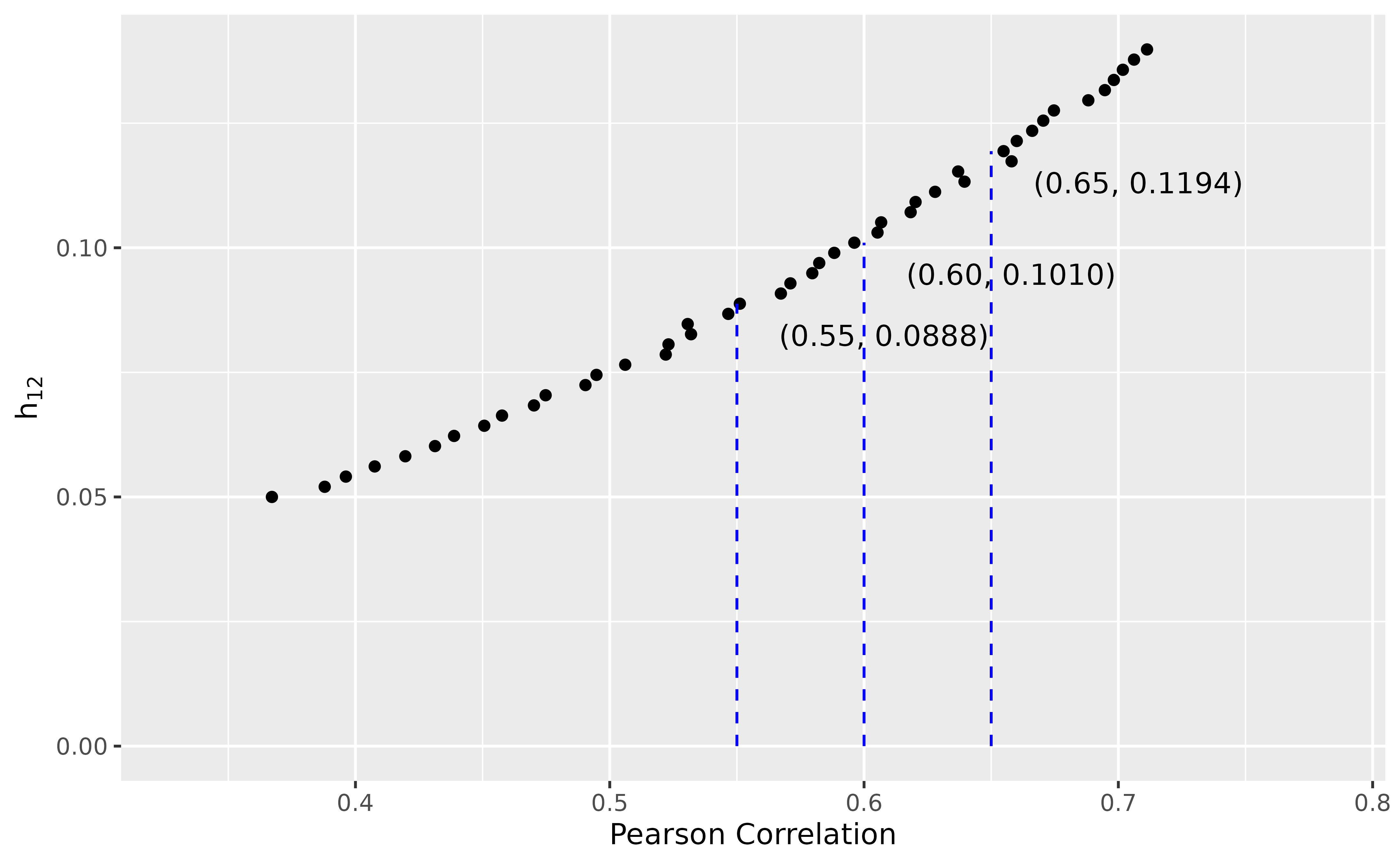
The result suggests that using , , and may achieve the desired specifications. To verify this, we simulate PFS and OS data using
pfs_and_os <- endpoint(name = c('pfs', 'os'),
type = c('tte', 'tte'),
generator = CorrelatedPfsAndOs3,
h01 = .11, h02 = .03, h12 = .10,
pfs_name = 'pfs', os_name = 'os')
pfs_and_osIf you are not familiar with the TrialSimulator
framework, you can generate PFS and OS directly by calling
CorrelatedPfsAndOs3():
dat <- CorrelatedPfsAndOs3(n = 1e6, h01 = .11, h02 = .03, h12 = .10)
head(dat, 2)
#> pfs os pfs_event os_event
#> 1 4.263915 12.997581 1 1
#> 2 4.257848 4.257848 1 1
## should be close to 0.6
with(dat, cor(pfs, os))
#> [1] 0.58961
## should be close to 5.0
with(dat, median(pfs))
#> [1] 4.965547
## should be close to 12.0
with(dat, median(os))
#> [1] 12.06319
with(dat, all(pfs <= os))
#> [1] TRUEFurther Discussion
In this vignette, we demonstrate how to derive the transition hazards from the medians of PFS and OS, along with their Pearson correlation coefficient. It is important to note that the correlation is computed based on data simulated from the specified hazard, and therefore can be substituted by any other measure that is relevant to the simulation objective.
For instance, one may simulate trial data for two treatment arms, each defined by their own PFS and OS medians and . Instead of using the correlation between simulated PFS and OS times, one could compute the correlation between the -statistics from the respective treatment comparisons of PFS and OS. This alternative approach may be more appropriate in scenarios where the joint behavior of test statistics, rather than raw survival times, is of primary interest—such as in the context of multiple testing or endpoint selection.